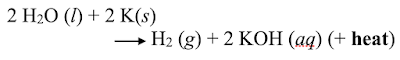Watch Faraday's Lecture 3, Products of Combustion
Read or Watch
• How Do Chemists Learn the Structures of Molecules?
Read and watch the materials on this page:
Molecules, and How Scientists Know
For now, just watch the first video "Molecules". If you find this interesting and want to explore further, watch more.
These videos are challenging, but just try to get the gist of them. If there are specific points that interest you, or you want to understand them better, ask about them in class, or by email.
• Chemical Equations
Convince yourself that each equation is balanced.
Potassium test for water:
Watch this video, which provides additional information about the water-potassium reaction. Watch for several examples of how properties of elements in the same column of the periodic table are related to each other.
• Read this poem:
"Identity", by A.R. Ammons This is a challenging poem. If you are interested by it, spend some time trying to interpret it. (What is an interpretation of a poem? Is formulating an interpretation anything like doing science?)
If you find "Identity" difficult to understand, this page features a discussion of the poem. You may not agree with all of it, but it includes an example of a defensible interpretation.
Submit Your questions using the instructions at the bottom of this page.
Your questions and comments help me to keep the course at your level.
••••••
Additional Resources (optional)
Commentary on Lecture 3
by the producers of the video series